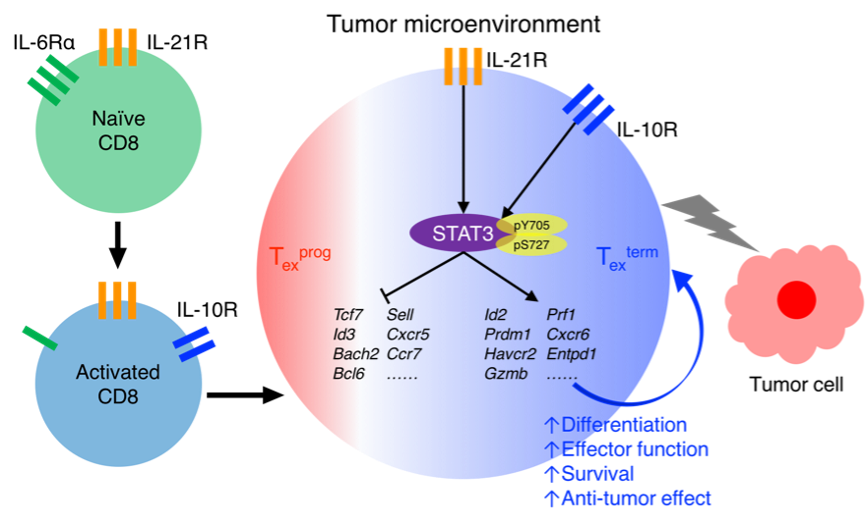
In cancer, persistent antigens drive CD8+ T cell differentiation into exhausted progenitor (Texprog) and terminally exhausted (Texterm) cells. However, how the extrinsic and intrinsic regulatory mechanisms cooperate during this process still remains not well understood. Here, we found that STAT3 signaling plays essential roles in promoting intratumor Texterm cell development by enhancing their effector functions and survival, which results in better tumor control. In tumor microenvironments, STAT3 is predominantly activated by IL-10 and IL-21, but not IL-6. Besides, STAT3 also plays critical roles in the development and function of terminally differentiated effector CD8+ T cells in acute infection. Mechanistically, STAT3 transcriptionally promotes the expression of effector function-related genes, while it suppresses those expressed by the progenitor Tex subset. Moreover, STAT3 functions in collaboration with BATF and IRF4 to mediate chromatin activation at the effector gene loci. Thus, we have elucidated the roles of STAT3 signaling in terminally differentiated CD8+ T cell development, especially in cancer, which benefits the development of more effective immunotherapies against tumors.
Subjects:
In acute infection and vaccination, effector CD8+ T cells develop into two subpopulations: one subpopulation is KLRG1+IL-7Rlow short-lived effector CD8+ T cells, also termed terminally differentiated effector CD8+ T cells, and another, KLRG1−IL-7Rhi memory precursors, which can subsequently develop into memory CD8+ T cells after the clearance of antigens (Joshi et al., 2007; Rutishauser et al., 2009). However, in cancer and chronic infection, persistent antigenic stimulation drives CD8+ T cells into an exhaustion state (McLane et al., 2019; Pauken and Wherry, 2015). Exhausted CD8+ T cells (Tex) are functionally distinct from effector and memory CD8+ T cells observed in acute infections and are characterized by elevated expression of inhibitory receptors (PD-1, LAG3, TIGIT, etc.), poor effector functions, altered epigenetic and transcriptional landscapes, and skewed metabolic status (McLane et al., 2019). Similar to the classification of effector CD8+ T cells, in cancer, tumor-specific Tex cells are mainly classified into two subpopulations, named progenitors (Texprog) and terminally differentiated CD8+ T (Texterm) cells (Miller et al., 2019; Siddiqui et al., 2019). Tumor-specific Texprog cells are stem-like and highly express TCF1 transcription factor, which persist long term and are capable of differentiating into Texterm cells for long-term tumor control (Miller et al., 2019; Siddiqui et al., 2019). A distinguishing feature of memory CD8+ T cells is their ability to rapidly expand and efficiently generate effector functions in response to antigen re-stimulation. Although Texprog cells are stem-like, their memory potentials are progressively lost and not at the levels of memory CD8+ T cells generated in acute infection (Angelosanto et al., 2012; Huang et al., 2022; Kaech and Cui, 2012; McLane et al., 2019). In contrast, tumor-specific TIM-3+ Texterm cells have characteristics of potent cytotoxicity, active proliferative capacity, and high-level apoptosis (Miller et al., 2019; Siddiqui et al., 2019). However, the transcriptional and epigenetic mechanisms underlying CD8+ T cell differentiation and maintenance, especially Texterm cells in cancer, remain elusive, and the cell-intrinsic and -extrinsic factors controlling Tex cell differentiation also need to be further identified.
T cell differentiation is generally regulated by three signals: signal 1 from antigen receptors, signal 2 from costimulatory or inhibitory molecules, and signal 3 from inflammatory cytokines (McLane et al., 2019). During chronic infection and cancer, persistent T cell receptor stimulation sustains the activation of NFAT and induces high-level expression of TOX and Nr4a1, which limit T cell function and promote T cell exhaustion (Chen et al., 2019; Khan et al., 2019; Liu et al., 2019; Martinez et al., 2015; Scott et al., 2019). In addition, elevated expression of coinhibitory receptors (e.g., PD-1 and CTLA-4) in Tex cells also enforces exhaustion, and the blockade of their signaling has shown clinical benefits in various human cancer types (Callahan et al., 2016; Kamphorst et al., 2017). Moreover, it has been reported that diminished TCR and costimulatory signaling in established chronic infection preferentially programs Tex cells into TCF1+ memory-like subset (Snell et al., 2018). Importantly, in addition to TCR and costimulatory/inhibitory molecules, Tex cell generation is also shaped by a broad range of inflammatory cytokines (McLane et al., 2019). Among them, JAK-STAT cytokines are critical in regulating T cell development in immune responses (Dong, 2021). For example, STAT1 is critical in promoting T cell expansion and antitumor response in cancer (Ryan et al., 2020). Consistently, STAT1 upstream cytokines, including type I IFN and IL-27, function to drive TIM-3+ Tex cell differentiation in both cancer and chronic infection (Chihara et al., 2018; Wu et al., 2016; Yang et al., 2012). In addition, IL-2–STAT5 axis functions as an inducer of exhaustion and promotes the generation of TIM-3+ Tex cells within tumors (Liu et al., 2021).
STAT3 is a cytoplasmic transcription factor belonging to the signal transducer and activators of transcription (STAT) family (Darnell, 1997). It can be phosphorylated and activated by multiple cytokines (e.g., IL-6, IL-10, IL-21, and IL-23), and it transcriptionally regulates the fate decisions of various CD4+ T lymphocyte lineages (Dong, 2021). The IL-6/IL-23–STAT3 axis induces RORγt expression during Th17 cell development (Chang et al., 2020; Yang et al., 2007), and the IL-6/IL-21–STAT3 pathway also promotes Bcl6 expression during Tfh cell differentiation (Nurieva et al., 2009; Xu et al., 2019). However, the role of STAT3 in regulating CD8+ T cell differentiation and function remains controversial and lacks mechanistic understanding. More importantly, its role in tumor-specific Tex cell development still remains unclear. STAT3 was long considered to promote memory CD8+ T cell development (Cui et al., 2011; Hanna et al., 2021; Olson and Jameson, 2011; Siegel et al., 2011). A previous study showed that Stat3 deficiency impaired the maturation and self-renewal of IL-7RhiKLRG1low memory CD8+ T cells in acute LCMV virus–infected mice (Cui et al., 2011). The development and maintenance of human central memory CD8+ T cells were also diminished in autosomal-dominant hyper-IgE syndrome patients with STAT3 mutations (Siegel et al., 2011). Moreover, in the chronic lymphocytic leukemia model, Stat3 deletion lowered the frequencies of memory-like PD-1intTCF-1+ CD8+ T cells (Hanna et al., 2021), and the leptin-induced STAT3 activation in obesity-driven breast cancer inhibited IFN-γ production and CD8+ T cell effector function (Zhang et al., 2020). However, contrary to these findings, another study showed that Stat3 deficiency decreased KLRG-1+ effector CD8+ T cells in HSV-1 infection (Yu et al., 2013) and reduced the expression of Blimp1, BATF, and Granzyme B in IL-21–stimulated CD8+ T cells in vitro (Xin et al., 2015). Thus, the role of STAT3 signaling in CD8+ T cell differentiation and function, especially in the context of tumor antigen–driven exhaustion, urgently needs to be clarified via a detailed mechanistic approach, and its upstream inducers within the tumors also need to be systematically identified.
In this study, we illustrated the function of STAT3 signaling in terminally differentiated CD8+ T cells, especially in cancer. Activated by IL-10 and IL-21, STAT3 played critical roles in promoting tumor-specific Texterm cell development and their effector functions in cancer. It was also intrinsically critical in promoting the development and function of terminally differentiated effector CD8+ T cells in acute infection. Mechanistically, STAT3 transcriptionally promoted the expression of Texterm-associated genes and inhibited that of Texprog genes, thus enforcing the Texterm transcriptional program. STAT3 functioned by epigenetically activating multiple Texterm-related gene loci. In addition, STAT3 cooperated with BATF and IRF4 to mediate Texterm cell differentiation. Altogether, this study has mechanistically clarified the role of STAT3 signaling in terminally differentiated CD8+ T cells, especially in cancer, which may benefit the development of novel cancer immunotherapies.

Copyright © 2017 Institute for Immunology Tsinghua University
Contact Address: Room D302, Medical Science Building, Tsinghua University, Beijing 100084, China
Tel: (86) 10-62776420 Fax: (86) 10-62776420
