On August 12, 2019, a research team led by Dr. Wanli Liu from School of Life Sciences, Institute for Immunology, Tsinghua University published an invited review “A PI(4,5)P2-derived “gasoline engine model” for the sustained B cell receptor activation” in Immunological Reviews. In this paper, they reviewed the studies that have contributed to uncovering the molecular mechanisms of PIP2-derived signaling amplification model. Based on these studies, they proposed a “gasoline engine model” in which the activation of B cell signaling inside the microclusters is similar to the working principle of burning gasoline within the engine chamber of a gasoline engine. They also discussed the evidences showing the potential universality of this model and future prospects.
B lymphocytes, as important participants in the antibody response, are involved in maintaining human health. The immune activation of B cell is a key step in the initiation of humoral immune response. B cells are activated by the recognition of pathogen through the cell surface B cell receptor (BCR), with instantaneous and highly dynamic properties, which has been a hot spot in immunological research for twenty years. After BCR antigen recognition, BCRs oligomerize to form signaling microclusters and accumulate at the antigen interface to form immunological synapse, which functions as the platform for the continuous transmembrane signal transduction and antigen gathering. The antigens’ B cells encounter may be rare, and the physical and chemical properties are diverse, which requires B cells with efficient signal transmembrane transduction mechanisms and intracellular signal amplification mechanisms to promote immune activation. In addition, PIP2 is a rare phospholipid on the plasma membrane, the mechanism of their regeneration after being depleted and their effects on the activation of B cells remains unexplained. In their previous study, various lipid biosensors, genetically modified cell lines, mouse and human primary B cells and PLCγ2 mutants from PLCγ2-associated immunodeficiency and dysfunction (PLAID) disease, combined with total internal reflection and confocal fluorescence microscopy based high-speed and high-resolution imaging systems were used to uncover the mechanism underlying the spatial and temporal metabolic regulation of PIP2 and its effects on B lymphocyte activation.
Their study had identified a signal amplification mechanism derived from PIP2 that is responsible for the sustained activation of B cells. After antigen recognition, phospholipase Cγ2 (PLCγ2) hydrolyzes PIP2 inside the BCR microclusters, and phosphatidylinositol 4-phosphate 5-kinase (PIP5K) catalyzes PIP2 regeneration outside the BCR microclusters. The signal transduction from inside to outside of the BCR microclusters is achieved by the fast diffusion of diacylglycerol (DAG), produced by the hydrolysis of PIP2 inside the BCR microclusters into outside the BCR microclusters, followed by the conversion of DAG to phosphatidic acid (PA) catalyzed by diacylglycerol kinase ζ (DGKζ), PA can then recruit and stimulate PIP5K to regenerate PIP2. Both the low density of PIP2 inside the BCR microclusters and the high density of PIP2 outside the BCR microclusters are important for the initiation of B cell activation.
The existence of a PIP2 spatial-temporal density gradient within the B cell immunological synapse during B cell activation shall be mediated by the PIP2 hydrolysis and synthesis feedback. PIP2 is efficiently depleted inside of the BCR microclusters but at the same time is also newly synthesized outside of the BCR microclusters. Moreover, the hydrolysis inside of the microclusters shows a positive feedback for the synthesis outside of the microclusters, through DAG diffused from the microcluster and then converted into PA to activate PIP5K. In return, PIP2 synthesized outside of the microclusters positively regulates its hydrolysis inside of the microclusters, since enrichment of PIP2 outside of the microclusters promotes the microcluster formation. Thus the PIP2 hydrolysis-derived feedback for its synthesis is a signaling amplification mechanism during the initiation of B cell receptor activation.
The critical step in the positive feedback loop of the PIP2 is the supply of PIP2 for PLCγ2. The PIP2 hydrolysis and synthesis derived BCR signaling amplification relies on two sources of PIP2. The first source is the basal PIP2 on the plasma membrane, which is hydrolyzed by PLCγ2 at early stages of initiation of B cell receptor activation. The second source of PIP2 is generated by the DAG-PA-DGKζ-PIP5K module from inside to outside of the BCR microclusters. The first intriguing question of this model is how the regenerated PIP2 outside of the microclusters can be utilized by PLCγ2 inside of the microclusters. The answer is likely the gradient based diffusion. In fact, they proposed that the high density of PIP2 outside of BCR microclusters can provide PIP2 for the PLCγ2 inside of the microclusters in a slow but persistent manner. This is very similar to the situation of the fuel supply in an actual working gasoline engine. To better illustrate this working model, an analogy of the gasoline engine is drawn: the lymphocyte signaling activation within the microclusters by consuming PIP2 is very similar to car engine functioning by burning gas (Figure). PIP2 generation outside of the microclusters just like the gas stored in the petrol tank. The gas is also supplied to the engine in a slow but persistent manner through a pipeline just similar to the PIP2 diffusion from outside of the microclusters (petrol tank) to inside of the microclusters (engine) (Figure).
Dr. Wanli Liu’s lab has been focusing on basic immunology research of B lymphocyte, integrating an interdisciplinary platform to investigate regulatory mechanisms of B cell activation and pathogenesis of related immune diseases. After the establishment of his own lab, Dr. Liu has made some achievements in pathogenesis study of B cell related autoimmune diseases (Science, J Exp Med, Cell Research, Leukemia2019a, Leukemia2019b, JACI, eLife 2019a); B cell activation regulation through lipid metabolism network (Cell, Science Immunology, Cell Reports, Nature Communications2015, Nature Communications2017); B cell activation regulation through BCR complex (Science Advances, PNAS, Chemical Science, eLife2019b); mechanical force sensitivity and threshold for the activation of BCR (J Cell Biol, Science Signaling, eLife2015, eLife2017, J Immunol, Eur J Immunol).
Chenguang Xu, a Ph.D. graduated from School of Life Sciences, Tsinghua University, is the first author, and Dr. Wanli Liu is the corresponding author of this review. They are funded by the National Science Foundation of China, and Institute of Immunology, Center for Life Science, Tsinghua University.
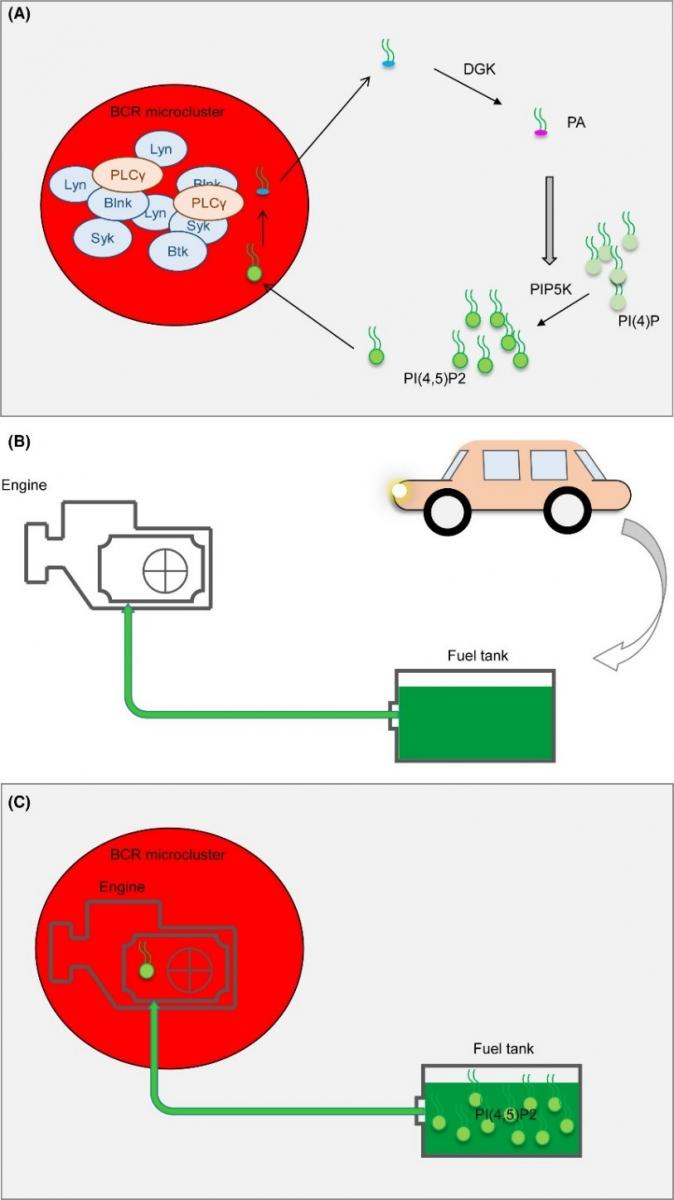
Figure. The “gasoline engine model” of BCR activation.
The analogy between the usage of PI(4,5)P2 in B cell activation and the usage of gasoline by an engine in an automobile. The BCR microclusters are the “engine” for B cell activation. PI(4,5)P2 is hydrolyzed inside of the microclusters, just as gasoline is burned in the engine. PI(4,5)P2 is stored outside of the microclusters as the gasoline is stored in the fuel tank. PI(4,5)P2 is provided slowly but persistently by slow diffusion like the gasoline is supplied from fuel tank to the engine also in a slow and persistent manner.
Link of the paper: https://onlinelibrary.wiley.com/doi/abs/10.1111/imr.12775

Copyright © 2017 Institute for Immunology Tsinghua University
Contact Address: Room D302, Medical Science Building, Tsinghua University, Beijing 100084, China
Tel: (86) 10-62776420 Fax: (86) 10-62776420
