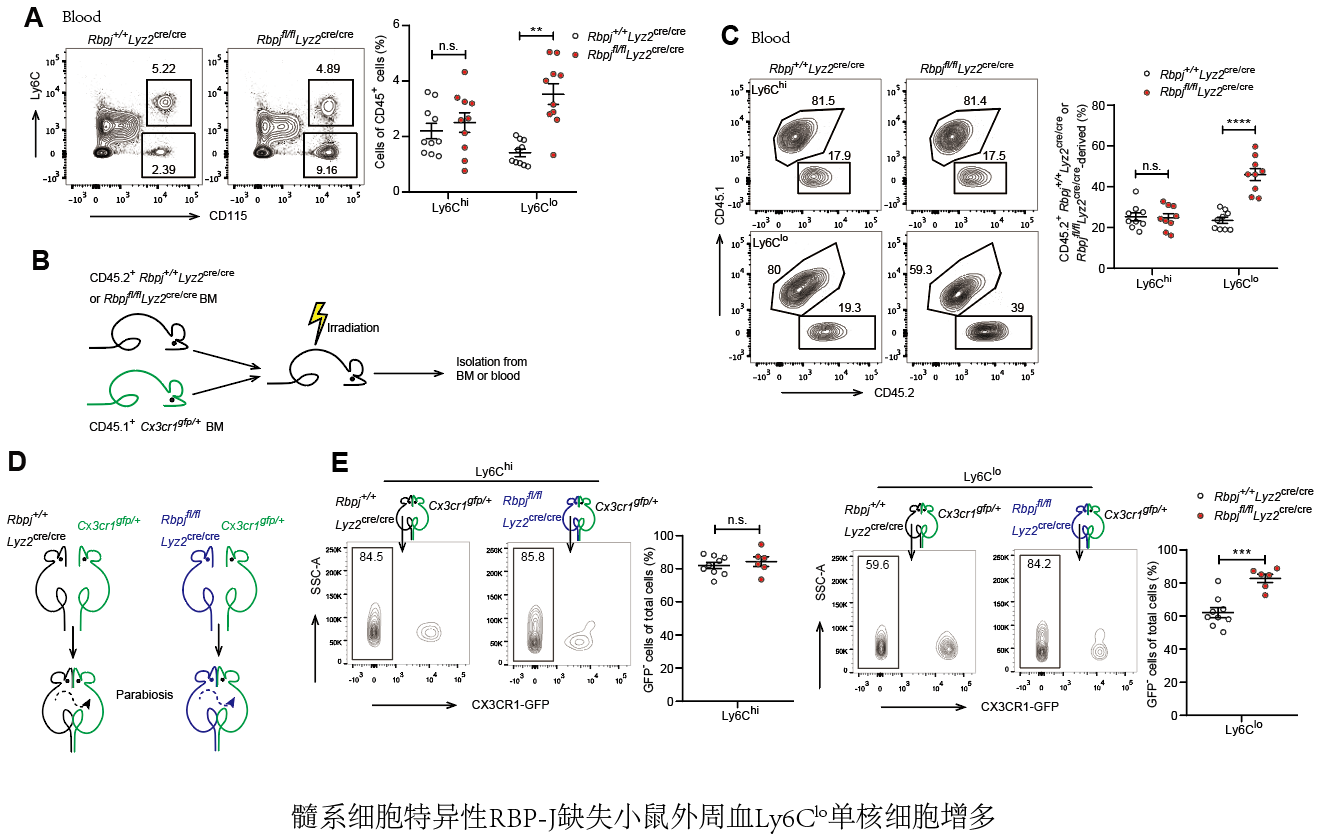
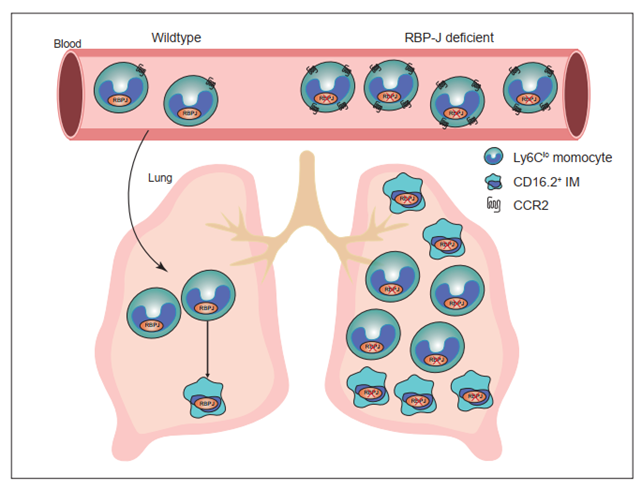
Notch-RBP-J signaling plays an essential role in maintenance of myeloid homeostasis. However, its role in monocyte cell fate decisions is not fully understood. Here we showed that conditional deletion of transcription factor RBP-J in myeloid cells resulted in marked accumulation of blood Ly6Clo monocytes that highly expressed chemokine receptor CCR2. Bone marrow transplantation and parabiosis experiments revealed a cell intrinsic requirement of RBP-J for controlling blood Ly6CloCCR2hi monocytes. RBP-J-deficient Ly6Clo monocytes exhibited enhanced capacity competing with wildtype counterparts in blood circulation. In accordance with alterations of circulating monocytes, RBP-J deficiency led to markedly increased population of lung tissues with Ly6Clo monocytes and CD16.2+ interstitial macrophages. Furthermore, RBP-J deficiency-associated phenotypes could be genetically corrected by further deleting Ccr2 in myeloid cells. These results demonstrate that RBP-J functions as a crucial regulator of blood Ly6Clo monocytes and thus derived lung-resident myeloid populations, at least in part through regulation of CCR2.
Monocytes are integral components of the mononuclear phagocyte system (MPS) that develop from monocyte precursors in the bone marrow(1). Several functionally and phenotypically distinct subsets of blood monocytes have been defined on the basis of expression of surface markers(2)-(5). In mice, Ly6Chi monocytes (also called inflammatory or classical monocytes) characterized by Ly6ChiCCR2hiCX3CR1lo exhibit a short half-life, and are recruited to tissue and differentiate into macrophages and dendritic cells(6). Ly6Chi monocytes are analogous to CD14+ human monocytes based on gene expression profiling(7). A second subtype of monocytes is called Ly6Clo monocytes (also termed patrolling monocytes or non-classical monocytes), which express high level of CX3CR1 and low level of CCR2, equivalent to CD14loCD16+ human monocytes. Ly6Clo monocytes are regarded as blood-resident macrophages that patrol blood vessels and scavenge microparticles attached to the endothelium under physiological conditions, and exhibit a long half-life in the steady-state(8)-(10). Ly6Clo monocytes may exert a protective effect by suppressing atherosclerosis in mice, while high levels of nonclassical monocytes have been associated with more advanced vascular dysfunction and oxidative stress in patients with coronary artery disease(11)-(13). In the inflammatory settings, Ly6Clo monocytes often show anti-inflammatory properties, yet also exhibit a proinflammatory role under certain circumstances(5),(9),(14)-(18). In addition, Ly6Clo monocytes have demonstrated protective functions in tumorigenesis, such as engulfing tumor materials to prevent cancer metastasis, activating and recruiting NK cells to the lungs(19),(20). While the functions of Ly6Clo monocytes are complex and sometimes contradictory depending on the animal models and experimental approaches, it is clear that they play a crucial role in both health and disease.
The generation and maintenance of Ly6Clo monocytes are regulated by several transcription factors, including Nr4a1 and CEBPβ(21),(22). The colony-stimulating factor 1 receptor (CSF1R) signaling pathway is important for the generation and survival of Ly6Clo monocytes, and neutralizing antibodies against CSF1R can reduce their numbers(23). Interestingly, recent studies have suggested that different subsets of monocytes may be supported by distinct cellular sources of CSF-1 within bone marrow niches, in which targeted deletion of Csf1 from sinusoidal endothelial cells selectively reduces Ly6Clo monocytes but not Ly6Chi monocytes(24). LAIR1 has been shown to be activated by stromal protein Colec12, and LAIR1 deficiency leads to aberrant proliferation and apoptosis in bone marrow non-classical monocytes(25). These findings further underscore the complexity of the mechanisms that regulate Ly6Clo monocyte, and suggest that multiple factors are involved in this process.
Recombination signal-binding protein for immunoglobulin kappa J region (RBP-J; also named CSL in humans) is commonly known as the master nuclear mediator of canonical Notch signaling(26). In the absence of Notch intracellular domain, RBP-J associates with co-repressor proteins to repress transcription of downstream target genes. In the immune system, the best studied functions of Notch signaling are its roles in regulating the development of lymphocytes, such as T cells and marginal zone B cells(26). Notch signaling also has been reported to regulate the differentiation and function of myeloid cells including granulocyte/monocyte progenitors, osteoclasts and dendritic cells (27)-(30). In osteoclasts, RBP-J represses osteoclastogenesis, particularly under inflammatory conditions(30). In dendritic cells, Notch-RBP-J signaling controls the maintenance of CD8- DC(28). In addition, Notch2-RBP-J pathway is essential for development of CD8-ESAM+ DC in the spleen and CD103+CD11b+ DC in the lamina propria of the intestine(29). However, the role of Notch-RBP-J signaling pathway in regulating monocyte subsets remains elusive. Here, we demonstrated that RBP-J controlled homeostasis of blood Ly6Clo monocytes in a cell intrinsic manner. RBP-J deficiency led to a drastic increase in blood Ly6Clo monocytes, lung Ly6Clo monocytes and CD16.2+ IM. Our results suggest that RBP-J plays a critical role in regulating the population and characteristics of Ly6Clo monocytes in the blood and lung.
Link:RBP-J regulates homeostasis and function of circulating Ly6Clo monocytes (elifesciences.org)

Copyright © 2017 Institute for Immunology Tsinghua University
Contact Address: Room D302, Medical Science Building, Tsinghua University, Beijing 100084, China
Tel: (86) 10-62776420 Fax: (86) 10-62776420
