A research article from Xiaoyu Hu’s Lab entitled “Hes1 attenuates type I IFN responses via VEGF-C and WDFY1” was published in Journal of Experimental Medicine on April 23rd, 2019. This work revealed that the transcription factor Hes1 functioned as a homeostatic negative regulator of type I IFNs for the maintenance of immune balance in the context of anti-viral immunity and autoimmune diseases.
Induction of type I interferons (IFNs) is a critical event for host defense during viral and bacterial infections, which can further activate downstream signaling pathways that lead to transcriptional induction of a wide range of IFN-stimulated genes (ISGs) encoding key immune effector molecules to elicit competent immune responses. However, excessive IFN production often acts as an amplifier of undesirable autoimmune and inflammatory responses and has been causally linked to pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE). Therefore, the magnitude and duration of IFN production must be precisely controlled to avoid IFN-related pathologies.
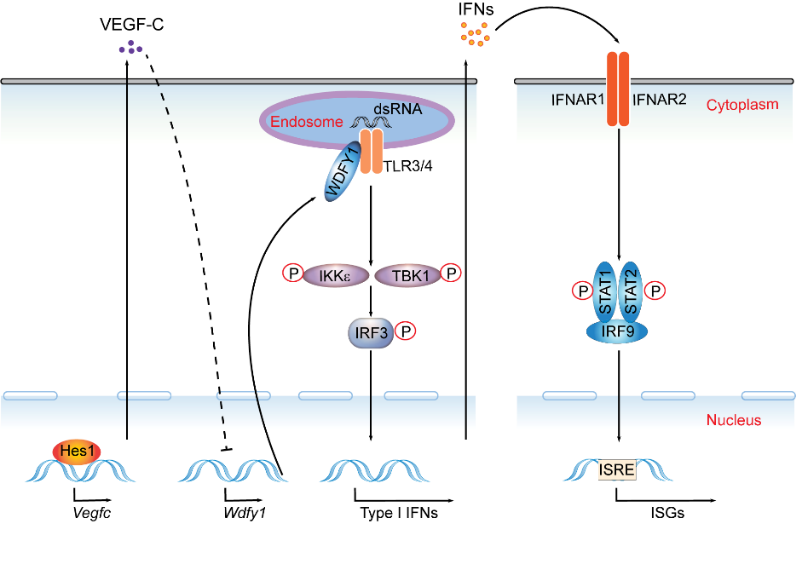
In this study, Hu and her colleagues found that transcription factor hairy and enhancer of split 1 (Hes1), a well known Notch target, suppressed production of type I IFNs and expression of IFN-stimulated genes. Functionally, Hes1-deficient mice displayed the heightened IFN signature in vivo, mounted enhanced resistance against encephalomyocarditis virus infection, and showed signs of exacerbated experimental lupus nephritis. Next, they investigated the underlying regulatory mechanisms and found that Hes1 did not suppress IFNs via direct transcriptional repression of IFN-encoding genes. Instead, Hes1 attenuated activation of TLR upstream signaling by inhibition of an adaptor molecule, WD-repeat and FYVE-domain–containing protein 1 (WDFY1). By genome-wide assessment of Hes1 occupancy, they further revealed that suppression of WDFY1 was secondary to direct binding and thus enhancement of expression of vascular endothelial growth factor C (VEGF-C) by Hes1, making Vegfc a rare example of a Hes1 positively regulated gene. This study identifying Hes1 as a suppressor of type I IFN production via VEGF-C and WDFY1 axis, together with the previous work from Dr. Hu’s group (Hu et al, Immunity, 2008; Xu et al, Nat Immunol., 2012; Shang et al, Nat Immunol., 2016; Zhang et al, Protein & Cell, 2019), systemically delineated the crucial role of Notch signaling components in regulation of innate immune responses.
Dr. Xiaoyu Hu and Dr. Yingli Shang (Shandong Agricultural University) served as the corresponding authors and a Ph.D. candidate Fei Ning is the first author. Researchers from Tsinghua University, Weill Cornell Medical College, and Wuhan University also contributed to this study. This work was funded by Ministry of Science and Technology of China, National Natural Science Foundation of China (NSFC), Tsinghua-Peking Center for Life Sciences, and the Institute for Immunology of Tsinghua University.
Link: http://jem.rupress.org/content/early/2019/04/22/jem.20180861

Copyright © 2017 Institute for Immunology Tsinghua University
Contact Address: Room D302, Medical Science Building, Tsinghua University, Beijing 100084, China
Tel: (86) 10-62776420 Fax: (86) 10-62776420
