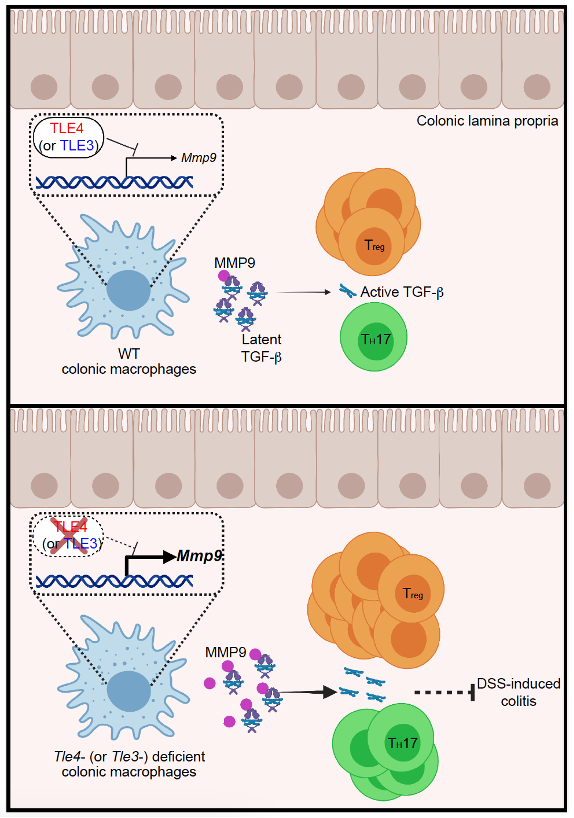
Colonic macrophages are critical for maintenance of cluster of differentiation 4 T helper (CD4+ T) cell homeostasis in the intestinal lamina propria. However, the mechanisms by which this process is regulated at the transcriptional level remain unknown. In this study, we found that the transcriptional corepressors transducin-like enhancer of split (TLE)3 and TLE4, but not TLE1 or TLE2, in colonic macrophages controlled homeostasis of CD4+ T-cell pool in the colonic lamina propria. Mice lacking TLE3 or TLE4 in myeloid cells exhibited markedly increased numbers of regulatory T (Treg) and T helper (TH) 17 cells under homeostatic conditions, rendering them more resistant to experimental colitis. Mechanistically, TLE3 and TLE4 negatively regulated matrix metalloproteinase (Mmp)9 transcription in colonic macrophages. Tle3 or Tle4 deficiency in colonic macrophages resulted in upregulated MMP9 production and thus enhanced latent transforming growth factor-beta (TGF-β) activation, which subsequently led to Treg and TH17 cell expansion. These results advanced our knowledge regarding the intricate crosstalk between the intestinal innate and adaptive immune compartments.
Cluster of differentiation 4 T helper (CD4+ T) cells represent an essential arm of the intestinal adaptive immune system, specializing in providing antigen-specific protective immunity. Whereas T helper (TH) 2 cells are largely absent from the normal intestine, TH1, TH17, and regulatory T (Treg) cells constitute the vast majority of CD4+ T cells in the intestinal lamina propria under homeostatic conditions[1], [2]. To prevent dysregulated CD4+ T-cell responses, a common feature of inflammatory bowel disease, numbers and activities of the intestinal CD4+ T-cell pool are tightly controlled by various mechanisms[1], [3], in which intestinal mononuclear phagocytes play essential roles. It has been widely accepted that unlike migratory dendritic cells (DCs), which prime and instruct differentiation of naive CD4+ T cells in intestine-draining lymph nodes by antigen presentation, nonmigratory macrophages maintain the integrity of polarized CD4+ T cells in the intestinal lamina propria4. However, the underlying regulatory programs are poorly understood.
The Drosophila corepressor Groucho interacts with DNA-binding repressors, especially Hairy-related proteins, to execute the transcriptional repression function during organism development5. Homologs or functionally and structurally related counterparts of the Groucho protein have been identified in fungi, plants, and mammals. Both the human and mouse genomes encode four full-length Groucho-related proteins, designated as transducing-like enhancer of split 1∼4 (TLE1∼4)5. Given the severe growth defects caused by global knockout (KO) of Tle16, Tle37, or Tle48 in mice, the functions of TLE proteins have been elucidated via cell type-specific gene targeting. In non-immune cells, TLE3 favors white adipocyte differentiation by repressing transcription of brown adipocyte-related genes[9], [10], and it supports pancreatic β cell development by repressing transcription of the hepatic program11. In the immune system, the TLE1/3/4 complex promotes CD8 lineage specification and identity consolidation by repressing transcription of CD4 signature genes12, while TLE4 inhibits inflammatory responses in macrophages by repressing transcription of interleukin (Il)6 and Il12p4013. These studies have implicated diverse functions of the evolutionarily conserved TLE family proteins in different cell types. The distinctive transcriptional and epigenetic profiles of tissue-resident macrophages[14], [15] prompted us to further investigate whether TLE proteins play a role in a particular cellular context, such as colonic macrophages, under homeostatic conditions.
Transforming growth factor-beta (TGF-β), a multifunctional cytokine, is involved in a plethora of immunological processes, such as immune cell development, modulation of inflammatory responses, and maintenance of immune tolerance[16], [17]. During naive CD4+ T-cell differentiation, TGF-β inhibits TH1 and TH2 cell differentiation yet favors Treg, TH17, and TH9 cell differentiation in cooperation with specific cytokines16. In the tissue microenvironment, TGF-β is secreted in a latent form and covalently binds to the extracellular matrix after secretion. However, TGF-β cannot exert its physiological function until it is liberated from the latent complex. Many factors have been reported to facilitate the release of active TGF-β through various mechanisms[17], [18], [19]. A previous study demonstrated that matrix metalloproteinase (MMP)9 and MMP2, cleave latent TGF-β on the surface of tumor cells and keratinocytes in a CD44-dependent manner20. Given the critical role of TGF-β in immune homeostasis, whether such a mechanism is active in immune cells under physiological conditions is unclear.
Here, we report that TLE3 and TLE4 in colonic macrophages function as key controllers of colonic TH17 and Treg cell homeostasis by suppressing MMP9-mediated TGF-β activation. These results identified two intestinal macrophage-intrinsic regulators of CD4+ T-cell homeostasis, adding another dimension to the delicate immune cell crosstalk in the intestinal tissue environment.

Copyright © 2017 Institute for Immunology Tsinghua University
Contact Address: Room D302, Medical Science Building, Tsinghua University, Beijing 100084, China
Tel: (86) 10-62776420 Fax: (86) 10-62776420
