On January 16th, 2020, Dr. Xiaoyu Hu’s group at the Institute for Immunology Tsinghua University published a research article entitled “the colonic macrophage transcription factor RBP-J orchestrates intestinal immunity against bacterial pathogens” in Journal of Experimental Medicine. This work revealed that transcription factor RBP-J in intestinal macrophages dynamically controls anti-bacterial immunity via interfacing with T cells and ILCs.
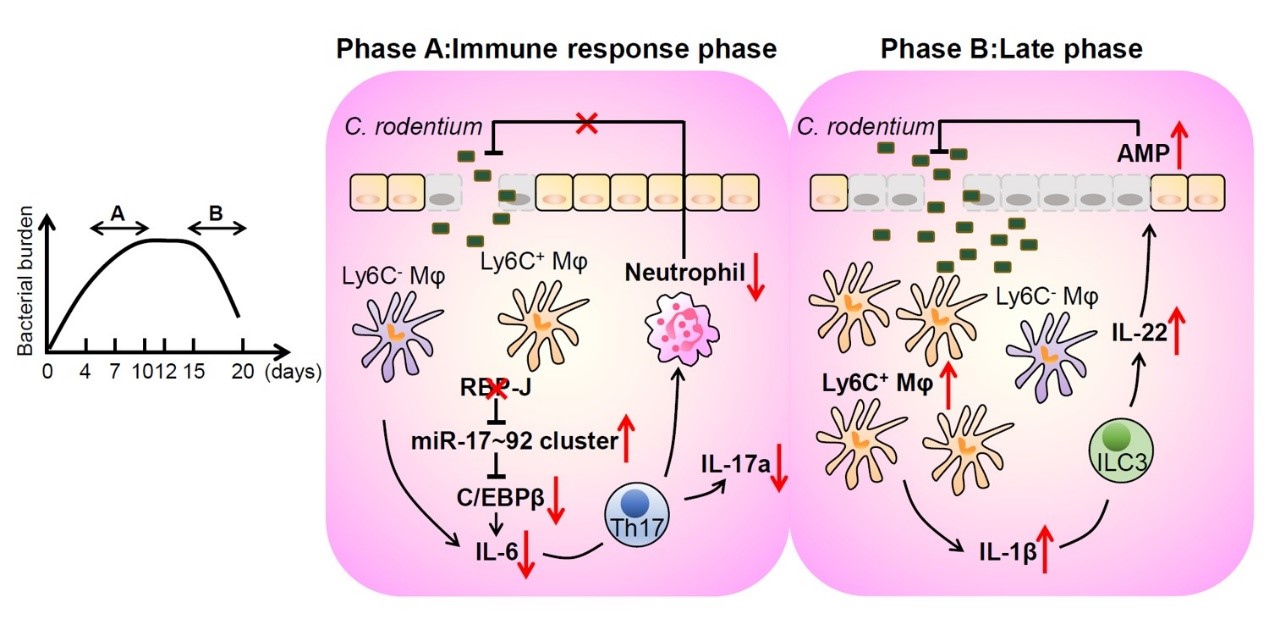
Macrophages play pleiotropic roles in maintaining the balance between immune tolerance and inflammatory responses in the gut. In this study, Hu and her colleagues identified transcription factor RBP-J as a crucial regulator of colonic macrophage-mediated immune responses against enteric pathogen Citrobacter rodentium. In the immune response phase, RBP-J promoted pathogen clearance by enhancing intestinal macrophage-elicited Th17 immune responses, which was achieved by maintenance of C/EBPβ-dependent IL-6 production through overcoming miRNA-17~92-mediated suppressive effects. RBP-J deficiency associated phenotypes could be genetically corrected by further deleting miRNA-17~92 in macrophages. In the late phase, non-eradicated pathogens in RBP-J KO mice recruited abundant IL-1β-expressing CD64+Ly6C+ colonic macrophages and thereby promoted persistence of ILC3-derived IL-22 to compensate for the impaired innate and adaptive immune responses, leading to ultimate clearance of pathogens. These results demonstrated that colonic macrophage-intrinsic RBP-J dynamically orchestrates intestinal immunity against pathogen infections via interfacing with key immune cells of T and innate lymphoid cell lineages. This study identifying RBP-J as a pivotal molecule in colonic macrophage-coordinated immune responses against C. rodentium, together with the previous work from Prof. Hu’s group (Hu et al, Immunity, 2008; Xu et al, Nat Immunol., 2012; Shang et al, Nat Immunol., 2016; Zhang et al, Protein& Cell, 2019;Ning et al, JEM, 2019), systemically delineated the crucial role of Notch signaling components in regulation of innate immune responses.
Dr. Xiaoyu Hu served as the corresponding authors and Ph.D. student Lan Kang is the first author. Researchers from Tsinghua University, Weill Cornell Medical College, and University College of London also contributed to this study. This study was supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Tsinghua-Peking Center for Life Sciences, Institute for Immunology at Tsinghua University and U.K. Royal Society.
Full text link: https://rupress.org/jem/article/217/4/e20190762/133608

Copyright © 2017 Institute for Immunology Tsinghua University
Contact Address: Room D302, Medical Science Building, Tsinghua University, Beijing 100084, China
Tel: (86) 10-62776420 Fax: (86) 10-62776420
